Acid Base Titration Lab Report
Citric acid is a polyprotic acid can release three H s that is a bit on the weak side ie tends not to ionize completely. Continue the titration to pH 42.
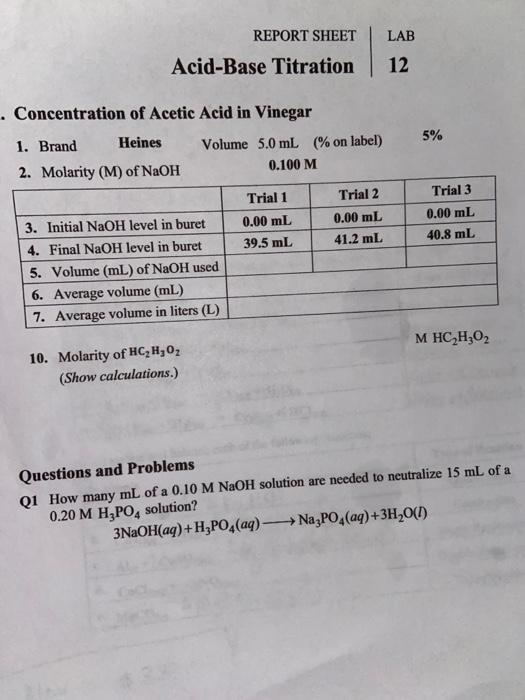
Solved Lab Report Sheet Acid Base Titration 12 Chegg Com
Acidbase titration is performed with a phenolphthalein indicator when it is a strong acid strong base titration a bromthymol blue indicator in weak acid weak base reactions and a methyl orange indicator for strong acid weak base reactions.
. AN does not represent the absolute acid concentration of the oil sample. Jewett with edits by R. This reaction is technically an oxidation-reduction reaction also called a redox reaction for short When ascorbic acid and starch are both in a.
With the digital titrator and sulfuric acid cartridge to pH 45. AN is the measure of acid concentration in a nonaqueous solution. Record the titrant used to this point as B.
CH3COOHaq NaOHaq - CH3COONaaq H2Ol By adding the sodium hydroxide which. Big 12 wrestling wiki. Titrate until the pH is 03 units below the starting point.
The neutralization reactions are. Acid-Base Titration Determination Of The Concentration Of Hydrochloric Acid Solution. The AN measurement detects both weak.
Apply stoichiometry to analyze titration data. Salt lake city water. In the first lab period the data to determine the enthalpy of reaction for Mg HCl and MgO HCl will be.
An example of titration using a starch indicator is the titration of vitamin C which is technically ascorbic acid. Calorimetry Lab ReportObjective. Write down the pH reading where you.
Edetic acid binds calcium and heavy metal ions forming soluble stable complexes which are readily excreted by the kidneys. 5 and the acid has a pH 5. Record titrant used to this point as A.
ASTM E223 Analysis of Sulfuric Acid. To standardise 02 M NaOH solution. The relative amounts of these materials can be determined by titration with acids.
This experiment essentially has three parts. Observe an acid-base reaction. ASTM D6209-13 Test to determine Gaseous and Particulate Polycyclic Aromatic Hydrocarbons in Ambient Air.
To determine the concentration of HCl solution. The base number is a measure of the amount of basic substance in the oil. HCl 1 365 365 H2SO4 2 981 490 CaCO3 2 100 500 AlOH3 3 780 260 For oxidationreduction reactions K is the number of moles of e- transferred per mole of oxidant or reductant in the balanced half-reaction.
You will use either HCl or H 2 SO 4 as your acid You may choose and NaOH as your base. Balanced half reaction K Fe3 Æ Fe 3 I2 Æ 2 I-2 2 S2O3 2-Æ S 4O6 2-1 Using Normality in titration calculations. STM D6174 Inorganic Sulfate in Surfactants by Potentiometric Lead Titration.
HCl aq NaOH. This experiment is designed to determine the molar concentration of acetic acid in a sample of vinegar by titrating it with a standard solution of NaOH. Neutralization between a strong acid HCl and a strong base NaOH is represented by H Cl- Na OH- Na Cl- H 2 O It is evident from the above equation that as NaOH solution is gradually added the H ion having high ionic conductance are replaced by Na having lower ionic conductance and hence the conductivity of the solution gradually decrease.
The standard unit of measure is mg KOHg. KHP is a weak acid and reacts with base in the following way. This results in a decrease in serum calcium levels.
To acquire the correct technique of titration. In the titration method phenolphthalein was used as an indicator which will be used to determine when the reaction reaches its endpoint. Ascorbic acid reacts with iodine to make dehydroascorbic acid and iodide ions.
If the initial pH is less than 45 record the initial pH value. A pH of 13. Enter the digits of titrant used as B.
Edetic Acid is the acid form of edetate a chelating agent with anti-hypercalcemic and anticoagulant properties. ASTM D6173 Various Anionic Surfactant Actives by Potentiometric Titration. In solution in fruit juices it lets a small portion of the H go however this.
To prepare a standard solution of oxalic acid. If the base is off the scale i. 51 New and used petroleum products can contain basic constituents that are present as additives.
This agent is also used as an anticoagulant for blood specimens and is applied as a. In a titration it is critical to know the exact concentration of NaOH in order to determine the concentration of the solution being tested. It is determined by the amount of potassium hydroxide KOH base required to neutralize the acid in one gram of an oil sample.
Clandestine chemistry is chemistry carried out in secret and particularly in illegal drug laboratoriesLarger labs are usually run by gangs or organized crime intending to produce for distribution on the black marketSmaller labs can be run by individual chemists working clandestinely in order to synthesize smaller amounts of controlled substances or simply out of. ASTM D6238-13 Test for Total Oxygen Demand in Water. Introduction Vinegar is a common household item containing acetic acid as well as some other chemicals.

Solved Section Titration Lab Report Sheet Table 1 Acid Base Chegg Com

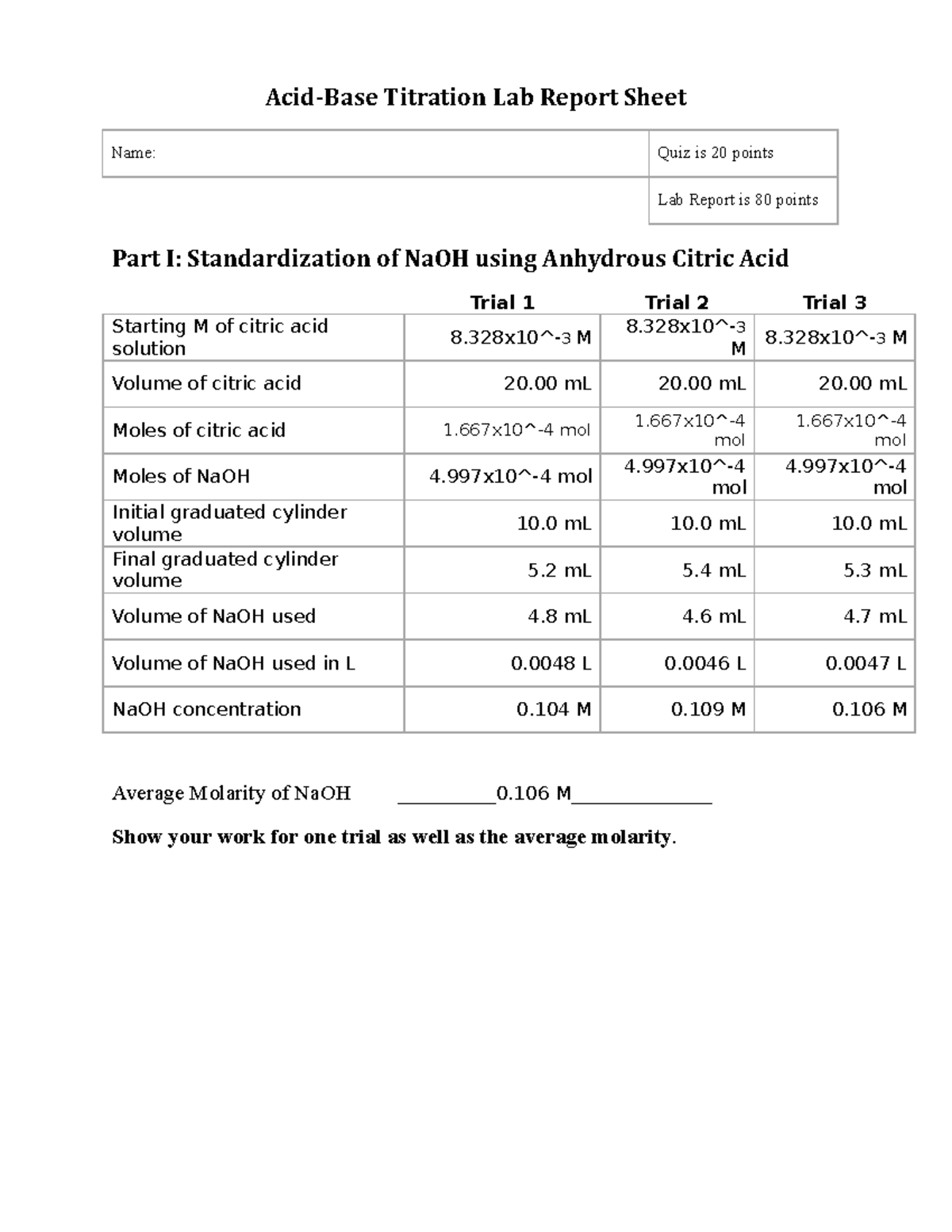
Acid Base Titration Lab Part Ii Determination Of Citric Acid In Unknown Impure Sample Trial 1 Studocu


No comments for "Acid Base Titration Lab Report"
Post a Comment